From Czuee Morey
Can blockchain be used in pharmacy and healthcare?
Blockchain is on everyone's lips these days and has also aroused interest in the pharmaceutical and healthcare industry. We at Wega Informatik recently evaluated blockchain from the perspective of both the technology and the potential applications for the pharmaceutical and healthcare industry.
Last week, Lorenz simplified the technical aspects of blockchain architecture and discussed how it relates to other existing peer-to-peer (P2P) technologies. If you haven't read this article yet, I encourage you to check it out first: https://www.wega-it.com/blockchain-beyond-the-hype
In short, Lorenz describes how the requirements of the problem should determine the choice of a solution - which does not necessarily have to be a blockchain! In fact, for many use cases, an existing and mature technology could be more useful than a blockchain, depending on trust between stakeholders, consensus, decentralization and other factors. He also discusses the limitations of blockchain technology such as scalability and the feasibility of tracking products with a limited lifecycle.
In this article, I will explore the potential use cases for blockchain in the pharmaceutical and healthcare industries and the considerations when implementing these solutions in more detail.
Applications of the blockchain
Blockchain could be applied in the pharmaceutical and healthcare industry in various areas from patient recruitment to insurance claim payments. Various start-ups, companies and even countries are working on blockchain implementations to solve various problems in this area. However, most of these solutions are currently still at the pilot or trial stage and it is still unclear what value blockchain can bring to this industry. However, the use cases can give us an idea of blockchain applications and the pros and cons of using this technology. I have listed some examples of different use cases in the market map below.
Although there is a wide range of business use cases, from a technology perspective, many of these applications would use essentially similar modules to implement identity verification, authorization and traceability. Let's take a closer look at three of these applications - drug supply chain integrity, medical data sharing and clinical trial data verification.

I. Maintaining the integrity of the pharmaceutical supply chain and preventing counterfeit medicines

The presence of counterfeit and contaminated drugs in the drug supply chain can be harmful to patients. The USFDA Drug Supply Chain Security Act (DSCSA), passed in 2013, mandates an electronic, interoperable system for identifying and tracing prescription drugs as they are distributed in the United States. This law mandates that by 2023, all parties involved must have full traceability down to the unit level throughout the supply chain from manufacturer to consumer and be able to identify and verify products suspected of being contaminated or counterfeit.
Previously, the pharmaceutical supply chain used the "Pedigree" (or e-Pedigree) system to track shipments. This required a declaration showing every previous sale, purchase or trade of the named medicine, including the date of these transactions and the names and addresses of all parties involved. Although this was initially a good basic system, it is still not completely foolproof against tampering and counterfeiting.
Therefore, a closed system with stricter controls to identify violations is required. A blockchain-based solution could be very helpful for medicines tracking as it fulfills the additional requirements of trust and security. It would make it easier to automatically detect and report counterfeit medicines and take the necessary mitigation measures in a timely manner. This investment also makes sense for manufacturers, as the ability to track and serialize every pharmaceutical package can minimize the cost of recalls and improve the accuracy of insurance chargebacks.
The implementation of a blockchain solution would require that all stakeholders such as manufacturers, distributors and dispensers are willing to adopt this technology. In addition, it is important to consider the limited product life cycle from manufacturing to sale, as maintaining the blockchain after the end of the life cycle would not add any value.
Several industry groups such as Mediledger (using Chronicled) are currently working on implementing this solution.
II Access to patient records/genomic data via blockchain

The applications for electronic patient databases, medical record storage and genomics data sharing are related because they primarily allow patients to own and share their data and determine who has access to it - clinicians, hospitals, researchers and/or pharmaceutical and biotech companies.
The existing solutions for transferring genomic or medical data use hashes to ensure that the data has been transferred in full. Encryption is used to ensure the security of the data and to grant access to certain parties. As patient data is highly sensitive, the use of blockchain could provide some additional security and ensure that medical records can only be accessed by certain people approved by the patient. In addition, patients can own and control their own data and be reimbursed for sharing it for research purposes.
The scalability of blockchain can be a problem as the number of patients and their data access requirements increase. Since a large amount of data cannot be stored on the blockchain, it is important that the data is stored accurately and securely. For example, if there is an error in a patient's blood type, this can be life-threatening in an emergency.
III Maintaining clinical trial data pathways for audits and ensuring data integrity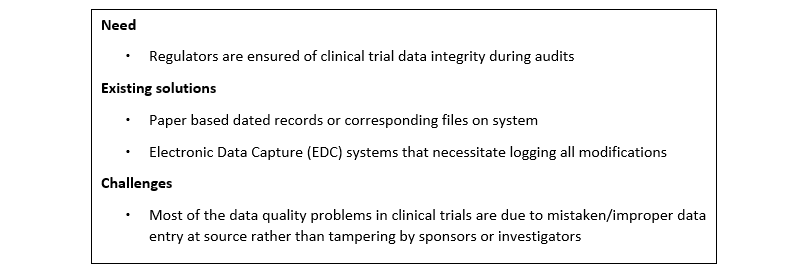
Data management in a clinical trial is complex. Data collection systems used for clinical trials must ensure that every data entry and change is carefully logged with the date and name of the person who entered it. In addition, the various persons involved in a clinical trial should only be able to access certain data sets. Regulatory authorities must ensure that clinical trial data accurately reflects the outcome of a trial and has not been manipulated. However, when auditing clinical trials, it is very difficult to penetrate all data sets to verify the authenticity of all data points.
If data access rights and data traceability are baked into a blockchain system, this can help ensure that clinical trial operations are conducted without tampering. This could also make regulatory audits much easier and faster.
However, there still needs to be a certain level of trust, as most of the data is entered manually during the clinical trial process. Any incorrect or improper data entry into the system would mean that the blockchain is protecting incorrect data. As other solutions such as eSource and IoT are implemented and data is captured directly from the source, a blockchain-based system would become more beneficial.
Summary
Blockchain is an interesting technology that offers various advantages over existing solutions, such as decentralization and security, which could greatly benefit certain areas of the pharmaceutical/healthcare industry. However, it is also important to think clearly about the problem requirements, the disadvantages of the technology, alternative technologies and possible risks in implementation.
I am a Consultant & Business Analyst at Wega Informatik AG in Basel. Wega Informatik is one of the leading IT service providers for pharma and life science in Switzerland.
If you have any questions or need support with implementing blockchain, please contact me at Czuee.Morey@wega-it.com
