L'Importance de l'archivage des données dans un environnement réglementé GxP
Ecrit par Piotr Kuchta et Robin Frey

Introduction
Dans les laboratoires d'analyse et de recherche scientifique industrielle, la génération de données analytiques complètes est un objectif principal pour servir les objectifs scientifiques. La génération de ces données dans l'analyse chromatographique est généralement prise en charge par des systèmes de données chromatographiques (CDS) complets tels que Thermo Scientific Chromeleon 7.3.2.
Différents fabricants proposent des solutions pour générer des données analytiques. En fonction de l'environnement dans lequel les données sont générées, la nécessité de répondre aux exigences réglementaires des différentes autorités devient importante.
Pour relever ces défis, une planification, une mise en œuvre et une validation correctes de l'environnement et du flux de travail sont nécessaires. Le CDS peut servir d'outil et de système opérationnel en remplissant son rôle dans le flux de travail de l'entreprise de manière robuste et rationalisée. Comme les laboratoires génèrent et accumulent des volumes importants de données provenant de diverses analyses chromatographiques, la nécessité d'un archivage efficace et sécurisé des données devient une préoccupation majeure.
Par exemple, lorsqu'un coffre-fort de Chromeleon atteint 90 % de sa capacité maximale de taille de base de données ou d'espace disque, le CDS en informe l'utilisateur en affichant une barre de notification rouge dans sa fenêtre principale.
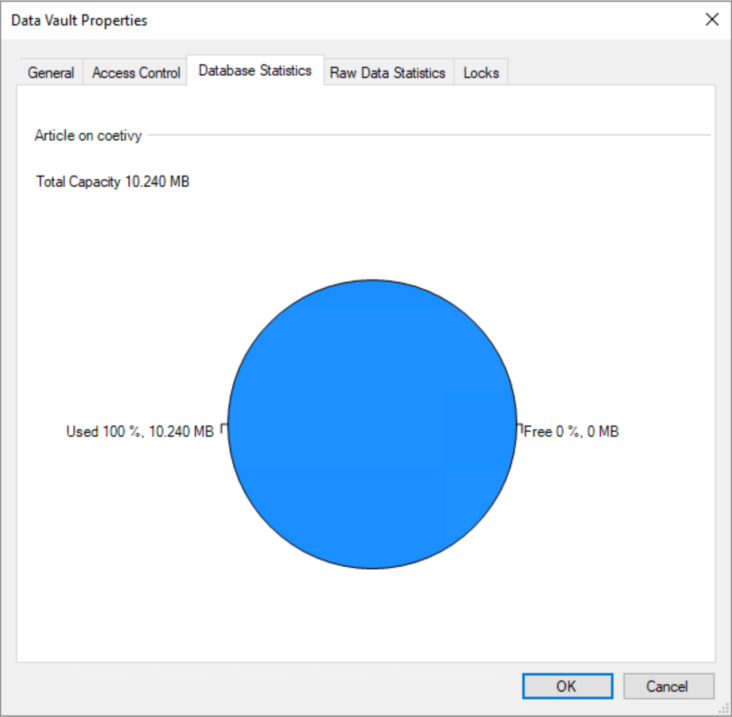
Ceci a été simulé dans Chromeleon 7.3.2 MUb et s'applique à toutes les versions actuelles de Chromeleon 7.
Chromeleon recommande donc à l'utilisateur de déplacer les séquences vers un autre coffre-fort ou de les supprimer définitivement.
Malheureusement, cette notification peut être ignorée par l'utilisateur et ne réapparaît qu'après l'ouverture d'une nouvelle session.
Si ce message de notification est ignoré suffisamment longtemps, la chambre forte peut finalement atteindre un point où la résolution du problème peut devenir fastidieuse ou simplement impossible sans une compréhension plus approfondie des bases de données et des outils supplémentaires disponibles uniquement en dehors de Chromeleon.
Dans Chromeleon, lorsque le coffre fort 100% de sa capacité, l'utilisateur ne pourra plus déplacer de séquences ou acquérir plus de données et ne pourra plus effectuer d'actions qui nécessitent que Chromeleon utilise une transaction de données avec la base de données. Dans ce cas, seule la base de données est pleine, tandis que l'espace disque est toujours disponible, mais la limite de la taille de la base de données est atteinte.
À ce stade, l'utilisateur voit apparaître le message d'erreur suivant : « L'opération a échoué car il n'y a pas assez d'espace disponible dans la base de données. S'il s'agit d'une édition SQL Server Express, la limite de taille intégrée peut être atteinte. Vous pouvez utiliser la console d'administration pour créer un nouveau coffre fort. »
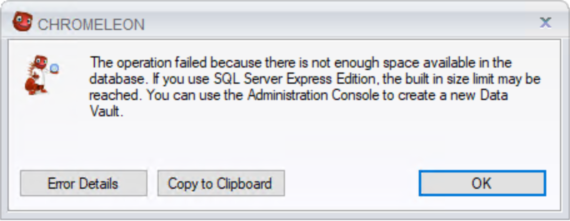
Les détails du message d'erreur permettent d'identifier la cause réelle du problème.
"Could not allocate space for object 'dbo.CR_AUDITTRAIL_ITEM'.'PK_CR_AUDITTRAIL_ITEM' in database because the 'PRIMARY' filegroup is full. Create disk space by deleting unneeded files, dropping objects in the filegroup, adding additional files to the filegroup, or setting autogrowth on for existing files in the filegroup.”
Cela se traduit par l'incapacité de Chromeleon à écrire l'action préférée dans le procès d'audit en raison de l'indisponibilité de la capacité du disque. Il propose également une solution en mentionnant la suppression des fichiers inutiles afin de faire de la place pour des fichiers supplémentaires dans le groupe de fichiers ou en choisissant de modifier les paramètres de croissance automatique pour le stockage en question.
Il est important de se rappeler que Chromeleon crée de nouvelles entrées dans la piste d'audit pour chaque action liée à la séquence qui est effectuée.
Pour expliquer davantage, même la suppression de données telles qu'une séquence de la corbeille de Chromeleon (pour au moins récupérer un peu d'espace) nécessite un stockage dans la base de données pour enregistrer le journal de la piste d'audit.
Un autre point qui mérite d'être mentionné est que les stations de travail et les contrôleurs d'instruments Chromeleon utilisent MS SQL Express Edition comme système de base de données, étant donné qu'il est fourni par le vendeur et qu'aucune licence supplémentaire n'est nécessaire. La limite est la capacité maximale de la base de données Microsoft SQL Server Express, qui est de 10 Go.
Si toutes les circonstances ci-dessus se produisent, la résolution d'un problème aussi complexe avec Chromeleon peut sembler impossible, mais il existe des moyens d'y remédier.
L'Importance de l'archivage des données dans un environnement réglementé GxP
L'exemple négatif montre que dans les environnements réglementés par les bonnes pratiques (GxP), l'archivage des données n'est pas seulement une bonne pratique, mais une exigence essentielle qui garantit non seulement le stockage adéquat des données, mais aussi la possibilité de les sauvegarder. Les réglementations GxP englobent des lignes directrices et des normes qui garantissent que les produits sont sûrs, qu'ils répondent aux normes de qualité et qu'ils sont produits de manière cohérente. En d'autres termes, les principes ALCOA+ sont appliqués à l'archivage des données pour soutenir la conformité réglementaire.
Principes d'ALCOA+
- Complet: Toutes les données, y compris les métadonnées, doivent être conservées. Pour les données archivées, cela signifie qu'il faut s'assurer que rien n'est perdu ou omis au cours du processus d'archivage.
- Consistent: Les données doivent être enregistrées de manière cohérente dans l'ensemble du système. Pour les archives, cela signifie qu'il faut maintenir un format et une structure cohérents pour le stockage des données.
- Durable: Les données doivent rester intactes et accessibles pendant toute la durée de leur conservation. Les données archivées doivent être stockées sur des supports durables et leur intégrité doit être régulièrement contrôlée.
- Disponible: Les données doivent être facilement accessibles à des fins d'examen et d'audit. Les données archivées doivent pouvoir être récupérées en temps utile, même des années après leur enregistrement initial.
Conserver trop de données dans le système sans les archiver correctement peut entraîner plusieurs problèmes
Différents problèmes se posent lorsque trop de données sont stockées sans une gestion appropriée :
- Ralentissement des performances : L'excès de données peut ralentir les performances du système, entraînant des temps de traitement plus longs pour l'extraction des données, l'analyse et la création de rapports.
- Complexité : Plus les données stockées dans le système sont nombreuses, plus la gestion des données devient complexe, ce qui complique l'organisation, la recherche et l'extraction d'ensembles de données spécifiques.
- Défis en matière d'audit : Lors des audits, un excès de données peut rendre difficile la localisation et la présentation rapide des informations requises, ce qui peut conduire à des résultats d'audit négatifs.
- Sauvegarde et récupération : Plus la quantité de données stockées est importante, plus les sauvegardes prennent du temps. La récupération de ces grandes quantités de données en cas de défaillance du système ou de catastrophe retarde le processus jusqu'à ce que les opérations puissent reprendre.
- Pas d'acquisition de données : Une base de données pleine ou des disques durs pleins sur les serveurs obligent l'utilisateur à agir sinon, dans le pire des cas, plus aucune donnée ne peut être acquise ou sauvegardée après une modification.
Meilleures pratiques pour atténuer ces problèmes
Pour atténuer ces problèmes, les organisations doivent mettre en œuvre des stratégies efficaces de gestion et d'archivage des données :
- Archivage régulier des données : mettre en œuvre un processus d'archivage de routine pour déplacer les données plus anciennes et moins fréquemment consultées vers des solutions de stockage sécurisées et conformes, telles qu'un autre coffre-fort de données.
- Politiques de conservation des données : Établir des politiques claires de conservation des données, conformément aux exigences réglementaires, afin de garantir que les données ne sont conservées que le temps nécessaire ; l'utilisation de tâches programmées dans Chromeleon peut servir à cette fin.
- Compression et optimisation des données : Utiliser des techniques de compression et d'optimisation des données pour réduire l'empreinte de stockage sans compromettre l'intégrité des données au niveau de la base de données.
- Gestion du cycle de vie des données : Employer des pratiques de gestion du cycle de vie des données pour s'assurer que les données sont correctement archivées, conservées et éliminées en fonction de leur valeur et des exigences réglementaires.
- Maintenance de la base de données : Compression de la base de données par la suppression des pages vides, la reconstruction des index et d'autres actions régulières.
- Stratégies de sauvegarde : Planifier des sauvegardes de la base de données et des fichiers journaux des transactions pour assurer la restauration de la base de données à un état de fonctionnement antérieur afin de garantir la continuité de l'activité.
En gérant efficacement le volume de données conservées dans les systèmes actifs, les organisations peuvent améliorer leurs performances, garantir la conformité, assurer la continuité des activités et réduire les coûts.
wega Informatik est heureux de vous aider à atteindre l'un des objectifs susmentionnés en tant que partenaire de planification, d'exécution, de documentation ou de validation. Forts d'une expérience de plusieurs années dans l'industrie pharmaceutique et sur d'autres marchés, nous fournissons ce type d'assistance et bien plus encore.
