Support for audits and inspections
Audits and authority inspections can have far-reaching organizational, production related and financial effects on a pharmaceutical company. In order to pass audits and inspections it is key that important measures must be taken: such as conducting trainings, preparations and continuous maintenance of readiness. The aim is to achieve a state of systems, documentation and processes that could be audited or inspected at any time.
The basis for continuous audit readiness is the awareness of all employees at all hierarchical levels of how important it is to comply with quality-related procedures and requirements in their daily work. Building on this, the system and process landscape must be implemented and operated in a validated state. This is the prerequisite for being able to reliably prepare for an audit or inspection.
Ready for inspections at any time
Identifying deviations and weaknesses before an official inspection is better and easier from both a financial and a reputation point of view. Through targeted mock inspections, often also through inexpensive and relatively simple assessments, improvement measures can be defined and then implemented.
The situation of an inspection is very stressful. It is therefore important to train employees on how to behave in this kind of a situation. In addition, professional preparation gives you a safe feeling during the inspection.
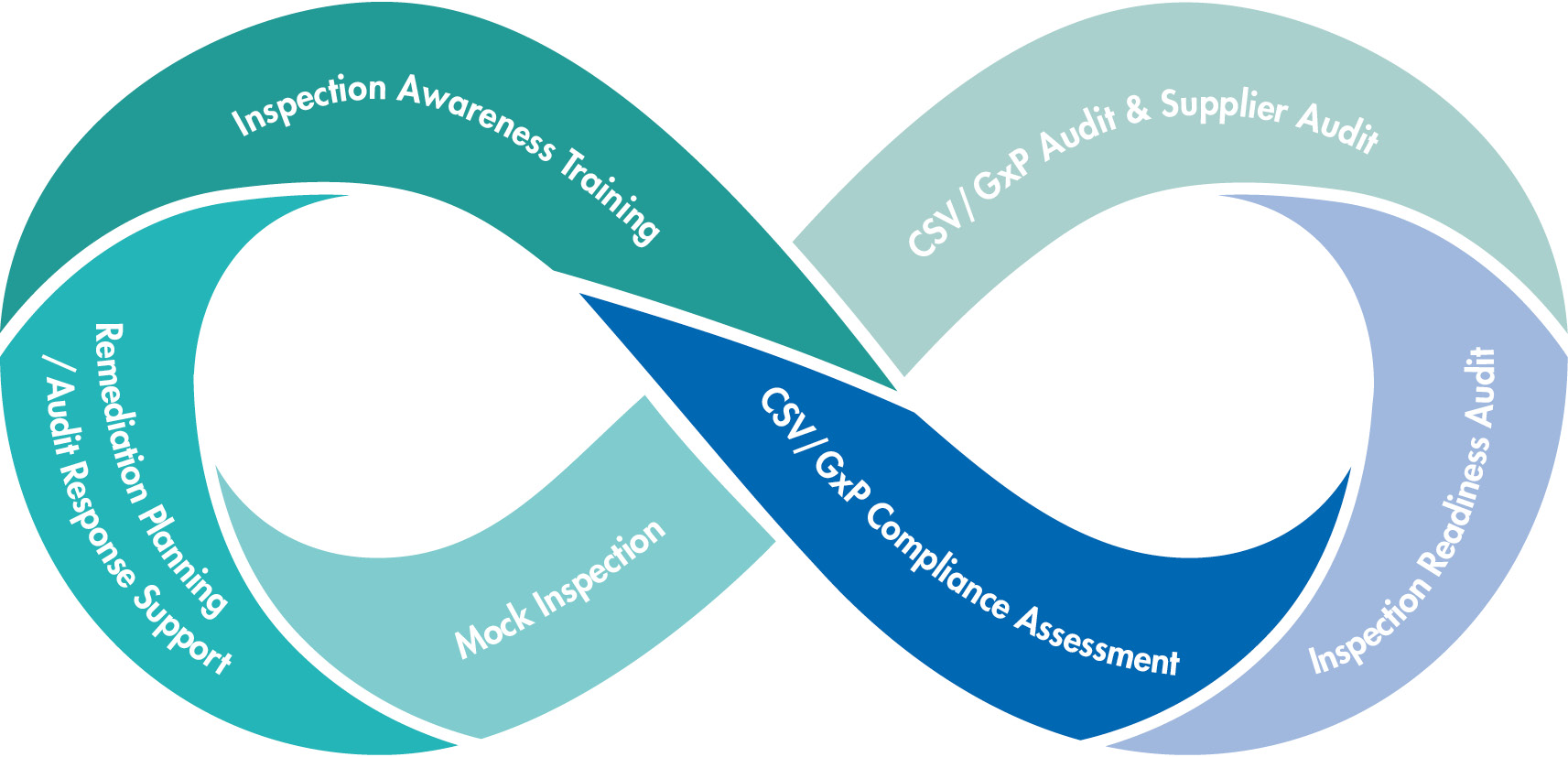
We support you in a variety of ways in preparing for regulatory inspections and audits. Customized training, assessments, supplier audits, formal CSV / GMP audits, which focus primarily on identifying weaknesses, through to mock inspections, in which the situation of an official inspection is simulated, give you more security to be ready for an inspection at any time and on an ongoing basis.
What we offer
- Company specific awareness trainings to coach employees at all levels
- Compliance assessments of computerized systems and processes
- Pre-inspection audits to assess the compliance state and readiness level
- Formal CSV/GxP audits, supplier audits, which primarily focus on the identification of weak points
- Mock inspections, in which the situation of an official inspection is simulated
- Support to plan remediation activities after inspections and to create audit responses
Contact us
